Research Interests
3. Organocatalysts with a pyridine nucleus
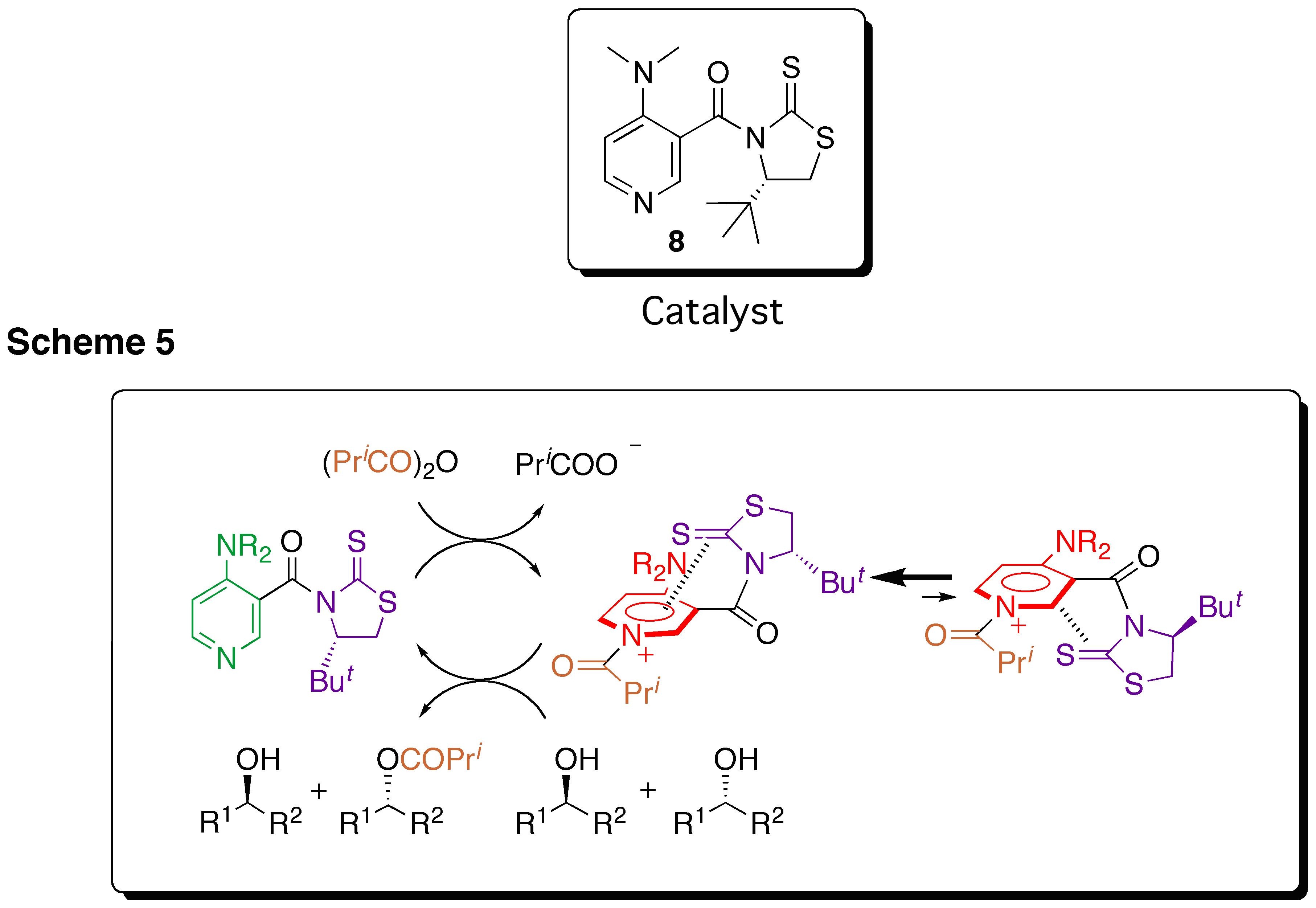
@We designed a new type of catalyst 8 possessing a thiocarbonyl group because the (C=S)EEEPy+ interaction would be effective for blocking one side of the pyridinium face. The characteristic feature of this catalyst is that the conformation is changed according to the acylation and deacylation steps as outlined in Scheme 5. Acylation of catalyst gives the conformationally fixed N-acylpyridinium salt through the (C=S)EEEPy+ interaction,14 which provides chiral environament around the N-acyl moiety. The reaction of racemic sec-alcohols with this intermediate would allow the enantio discrimination to give the corresponding chiral esters with recovery of the catalyst. The recovered catalyst with the restored conformational freedom is smoothly available for the next cycle. We anticipated that this conformational switch process would satisfy both the selectivity and reactivity.
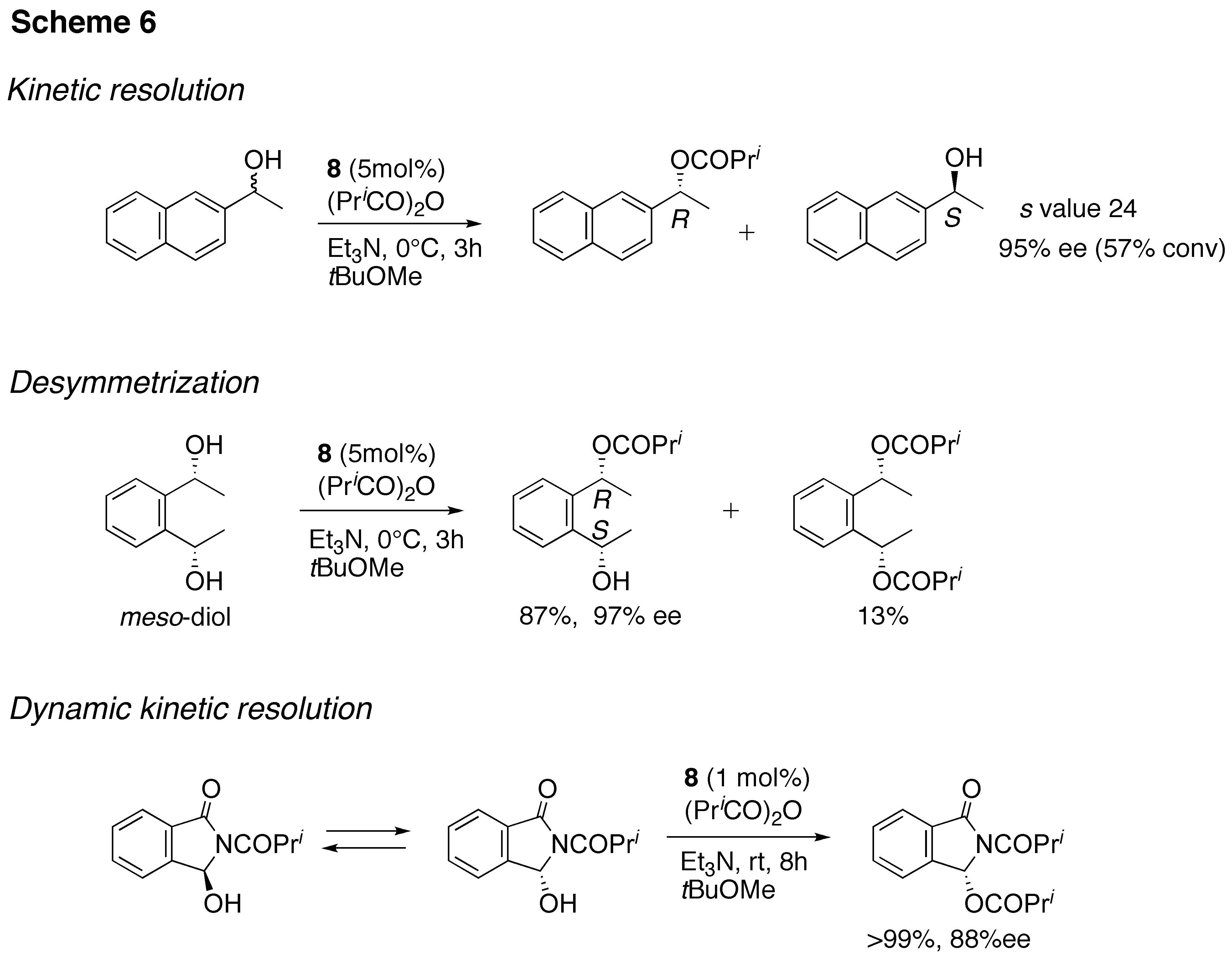
Kinetic resolution of 1-(2-naphthyl)ethanol with isobutyric anhydride was performed in in 95% ee in the presence of 5 mol % of catalyst 8 in toluene at 0C. This method can be applicable to a variety of sec-alcohols. Asymmetric desymmetrization of meso-diols and dynamic kinetic resolution of a hemiaminal were also achieved using the same catalyst as shown in Scheme 6.
1. Y. Mori and S. Yamada, gContribution of Cation-pi Interactions in Iminium Catalysish, Molecules, 2012, 17, 2161-2168
2. S. Yamada and J. S. Fossey, gNitrogen cation-pi interactions in asymmetric organocatalytic synthesish, Org. Biomol. Chem., 2011, 9, 7257-7580
3. S. Yamada, K. Yamashita, gDynamic Kinetic Resolution of Hemiaminals Using a Novel DMAP Catalysth, Tetrahedron Lett., 2008, 49, 32-35
4. S. Yamada, T. Misono, Y. Iwai, A. Masumizu, Y. Akiyama, gA New Class of Pyridine Catalyst Having a Conformation Switch System: Asymmetric Acylation of Various sec-Alcoholsh, J. Org. Chem., 2006, 71, 6872-6880
5. S. Yamada, T. Misono, Y. Iwai "Kinetic resolution of sec-alcohols by a new class of pyridine catalysts having a conformation switch system" Tetrahedron Lett., 2005, 46, 2239-2242
6. S. Yamada, T. Misono, S. Tsuzuki "Cation-p interaction of a Thiocarbonyl Group and a Carbonyl Group with a Pyridinium Nucleus" J. Am. Chem. Soc., 2004, 126, 9862-9872
@
1. Face-selective addition reactions
2. Stereoselective [2+2] photocyloaddition
TOP
3. Organocatalysts with a pyridine nucleus
@We designed a new type of catalyst 8 possessing a thiocarbonyl group because the (C=S)EEEPy+ interaction would be effective for blocking one side of the pyridinium face. The characteristic feature of this catalyst is that the conformation is changed according to the acylation and deacylation steps as outlined in Scheme 5. Acylation of catalyst gives the conformationally fixed N-acylpyridinium salt through the (C=S)EEEPy+ interaction,14 which provides chiral environament around the N-acyl moiety. The reaction of racemic sec-alcohols with this intermediate would allow the enantio discrimination to give the corresponding chiral esters with recovery of the catalyst. The recovered catalyst with the restored conformational freedom is smoothly available for the next cycle. We anticipated that this conformational switch process would satisfy both the selectivity and reactivity.

Kinetic resolution of 1-(2-naphthyl)ethanol with isobutyric anhydride was performed in in 95% ee in the presence of 5 mol % of catalyst 8 in toluene at 0C. This method can be applicable to a variety of sec-alcohols. Asymmetric desymmetrization of meso-diols and dynamic kinetic resolution of a hemiaminal were also achieved using the same catalyst as shown in Scheme 6.

1. Y. Mori and S. Yamada, gContribution of Cation-pi Interactions in Iminium Catalysish, Molecules, 2012, 17, 2161-2168
2. S. Yamada and J. S. Fossey, gNitrogen cation-pi interactions in asymmetric organocatalytic synthesish, Org. Biomol. Chem., 2011, 9, 7257-7580
3. S. Yamada, K. Yamashita, gDynamic Kinetic Resolution of Hemiaminals Using a Novel DMAP Catalysth, Tetrahedron Lett., 2008, 49, 32-35
4. S. Yamada, T. Misono, Y. Iwai, A. Masumizu, Y. Akiyama, gA New Class of Pyridine Catalyst Having a Conformation Switch System: Asymmetric Acylation of Various sec-Alcoholsh, J. Org. Chem., 2006, 71, 6872-6880
5. S. Yamada, T. Misono, Y. Iwai "Kinetic resolution of sec-alcohols by a new class of pyridine catalysts having a conformation switch system" Tetrahedron Lett., 2005, 46, 2239-2242
6. S. Yamada, T. Misono, S. Tsuzuki "Cation-p interaction of a Thiocarbonyl Group and a Carbonyl Group with a Pyridinium Nucleus" J. Am. Chem. Soc., 2004, 126, 9862-9872
@
1. Face-selective addition reactions
2. Stereoselective [2+2] photocyloaddition
TOP